ml-cvd-prediction
Midterm Report
Introduction
Cardiovascular disease (CVD), especially coronary heart disease (CHD), accounts for a major portion of global mortality 1. This has led to scientists collecting vast amount of data related to heart-disease and other conditions. With this data available, machine learning algorithms can better predict patients who are developing various kinds of diseases ranging from Diabetes to CVD 2. Research into which supervised learning techniques are best for CVD prediction is still ongoing into 2024 3, but we wish to also use this data to further develop unsupervised learning techniques since they can help us predict the disease without any labels.
We are exploring these two datasets:
- Cardiovascular Heart Disease Dataset from the Mendeley database
- Heart Disease Cleveland Dataset from the UC Irvine Machine Learning Repository
Both databases contains 12 features and a target variable specifying whether or not the patient was diagnosed with heart disease. They have 6 nominal values and 6 numeric values including age, blood pressure, and cholestrol levels.
Problem Definition
We want to use machine learning models to predict if someone has cardiovascular disease from various health metrics. Most of the prior studies 4 focused on supervised learning algorithms for making predictions; however, our project will focus on both unsupervised and supervised learning for more comprehensive results.
Methods - Data Processing
Data Cleaning
Some feature values, such as number of major vessels, are missing, so we decided to take the the mean from the rest of the column to fill in these missing values. We chose to use the ceiling of the mean specifically so we would not underestimate disease risk.
In addition, we combined the Mendeley and Cleveland datasets into one– resulting in 1303 samples in total, 303 from the Cleveland dataset and 800 from the Mendeley dataset. This is a table of all the features and what they mean:
| Feature | Values |
|---|---|
| age | years |
| gender | 0: female, 1: male |
| chestpain | 0: typical angina, 1: atypical angina, 2: non-anginal pain, 3: asymptomatic |
| restingBP | 94–200 mm/HG |
| serumcholestrol | 85-602 mg/dl |
| fastingbloodsugar | 0, 1 > 120 mg/dl |
| restingrelectro | 0: normal, 1: ST-T wave abnormality, 2: probable or definite left ventricular hypertrophy by Estes’ criteria |
| maxheartrate | 71 - 202 |
| exerciseangia | 0: no, 1: yes, whether exercise-induced angina is present |
| oldpeak | 0-6.2, indicates exercise-induced ST-depression relative to the rest state |
| slope | 1: upsloping, 2: flat, 3: downsloping, slope of the ST segment during peak exercise |
| noofmajorvessels | 0-3 |
For our target variable, 1 represents the presence of heart disease and 0 represents the absence of it. Using data from both datasets, we were able to construct a dataset that had an even number of patients from both target cases, with presence of heart disease being slightly overrepresented.
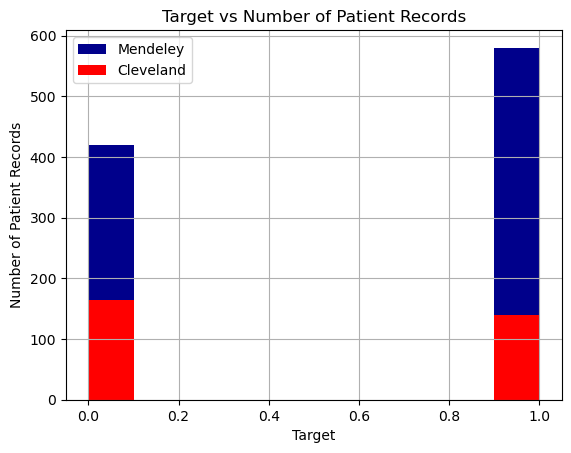
We also found that the patients represented in the Cleveland dataset are on average healthier than those in the Mendeley dataset (lower cholestrol, lower max heart rates, etc.), so combining the datasets makes an overall more representative dataset of different patient types. This will help prevent overfitting when we run our machine learning algorithms. It also simplifies the number of dataset we have to apply machine learning algorithms to.
PCA Feature Reduction
We realized that using all 12 features caused some of our models to be achieving low scores. Thus, we decided to apply PCA from Ski-Kit Learn to reduce the dataset’s dimensionality while preserving as much information as possible. PCA helped identify the main patterns in the data by transforming the original features into new, uncorrelated components.
Result shows that the first few principal components capture the majority of the variance:
PC1 captures ~17.1% of the variance. PC2 captures ~12.4%. PC3, PC4, and PC5 each capture between 8-11%. The rest of the components capture less but together, the components used explain around 95% of the dataset’s variance, meaning they retain most of the information in fewer dimensions. Feature Influence:
PC1 is most influenced by PCT, noofmajorvessels, and slope, suggesting these features contribute significantly to the data’s overall structure. PC2 focuses on chestpain, oldpeak, and age, capturing another aspect of the data. PC3 and PC4 also capture unique feature combinations, with PC3 highlighting gender and serumcholestrol, and PC4 focusing on age and maxheartrate.
Visualization Insights: The 2D scatter plot of PC1 vs. PC2 shows how observations cluster or separate in this reduced space, which can reveal patterns or relationships not easily visible in the high-dimensional data.
We found that PCA effectively reduced the dataset’s complexity, retaining the main data structure and revealing which feature combinations contribute most to each principal component. This reduction will make further analysis or modeling more efficient and focused on the most informative aspects of the data.
Methods - Machine Learning Model
Decision Tree Classifier (Supervised Learning)
We used Decision Tree Classifier from Sci-Kit Learn to train our model. Decision Tree Classifier was chosen because we could easily visualize in a tree what features help determine the predicted label as it creates a clear cutoffs for a binary outcome. For this model, we directly used all features after data cleaning to train our model.
KMeans (Unsupervised Learning)
We used KMeans from Sci-Kit Learn to train our model. KMeans is able to quickly converge to k cluster centroids and find out the typical values of features for a group that have cardiovascular diseases, making results highly interpretable. For our project, we first tried using all features with KMeans, then later with partial features after PCA to evaluate if a better model can be trained. We also tried various values of K to determine the best outcome for a specific number of features.
GMM (Unsupervised Learning)
We used GMM from Sci-Kit Learn to train our model. GMM is able to quickly converge to a given number of components and find out the typical values of features for a group that have cardiovascular diseases, making results highly interpretable. For our project, we first tried using all features with GMM, then later with partial features after PCA to evaluate if a better model can be trained. We also tried various values for the number of components to determine the best outcome for a specific number of features.
DBSCAN (Unsupervised Learning)
We used DBSCAN from Sci-Kit Learn to train our model. DBSCAN is able to group together closely connected points and separates loosely connected points, allowing for clustering patients that have shared risk factors. For our project, we first tried tuning the parameters necessary for performing DBSCAN. Currently, we are trying to optimize the DBSCAN performance accuracy.
Results and Discussion
Decision Tree Classifier
To fine-tune our decision tree classifier, we first decided on the best max depth. To better understand how a decision tree works with our data, we visualized what the tree looked like with a max depth of 3. This decision tree makes sense as values such as low chestpain would indicate that a patient does not have the diease.
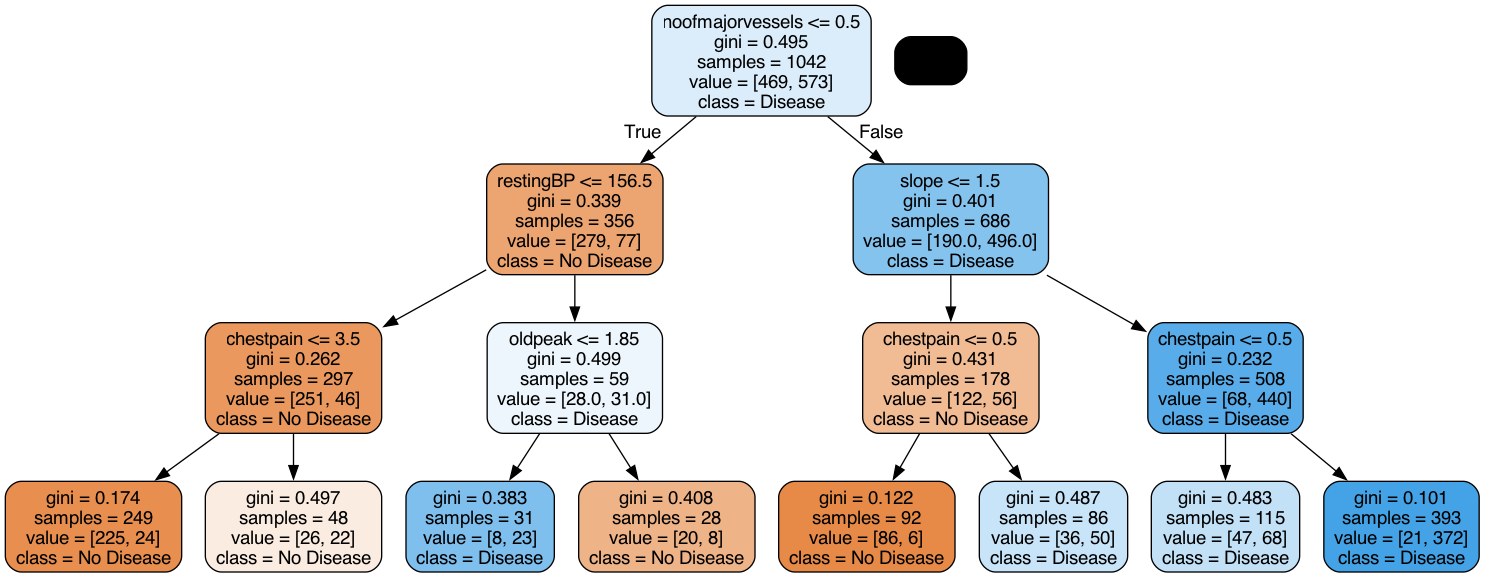
Then, we incremented the depth all the way to 15, and found that a depth of 7 yielded the highest accuracy
We performed a similar experiment for Min Samples Leaf and found that a value of 2 yields the highest accuracy.
With these changes, we got the following score for decision tree. Accuracy: 0.9119, F1 Score: 0.9187, Precision: 0.9489, Recall: 0.8904. Almost all the scores were above 90%, which shows that decision tree is an effective model to use for this dataset.
Here’s the confusion matrix for our predictions:
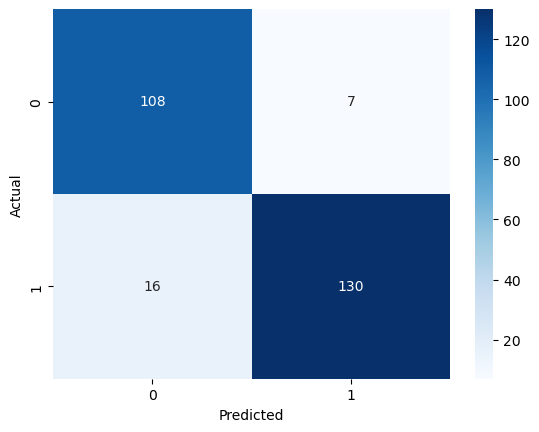
As we can see, there are more false negatives than false positives. This is why our recall score is lower and precision is higher. There is a trade-off between maximizing precision and recall, so we would favor this model if we were looking to ensure that patients who actually do not have heart disease do not get mislabeled.
For next steps, we can potentially feed in features extracted from PCA or another feature reduction method to see if a better model can be trained.
KMeans
We first trained KMeans through all features and tested with the amount of cluster k. We were able to see that with k=2 clusters, the model performed the best.
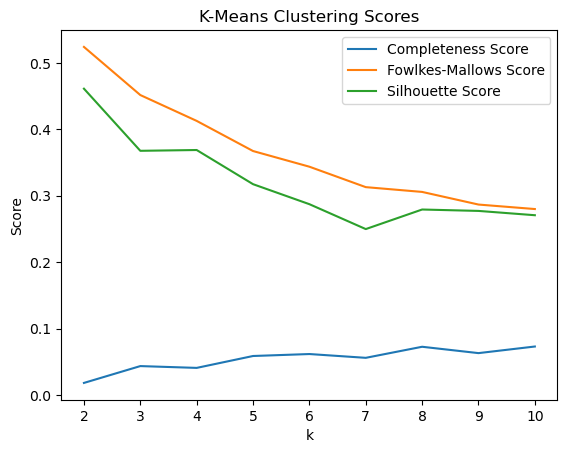
However, we wondered if the high dimensionality caused such a low high score. Thus, we decided to extract information from PCA to get a few features to use. From PCA, we are able to see that a few features appear frequently accross the tops of each PC, including
- Fasting Blood Sugar: Appears in PC1, PC6, PC7, PC9, PC10, and PC11.
- Resting BP: Appears in PC1, PC4, PC6, PC8, PC9, and PC11.
- Chest Pain: Appears in PC2, PC3, PC5, PC7, PC8, and PC11.
- Age: Appears in PC2, PC4, PC5, and PC7.
- Serum Cholesterol: Appears in PC1, PC3, PC9, and PC11.
We tested KMeans with the top 2, 3, 4, and 5 features, along with various amount of cluster k to see which one performs the best.
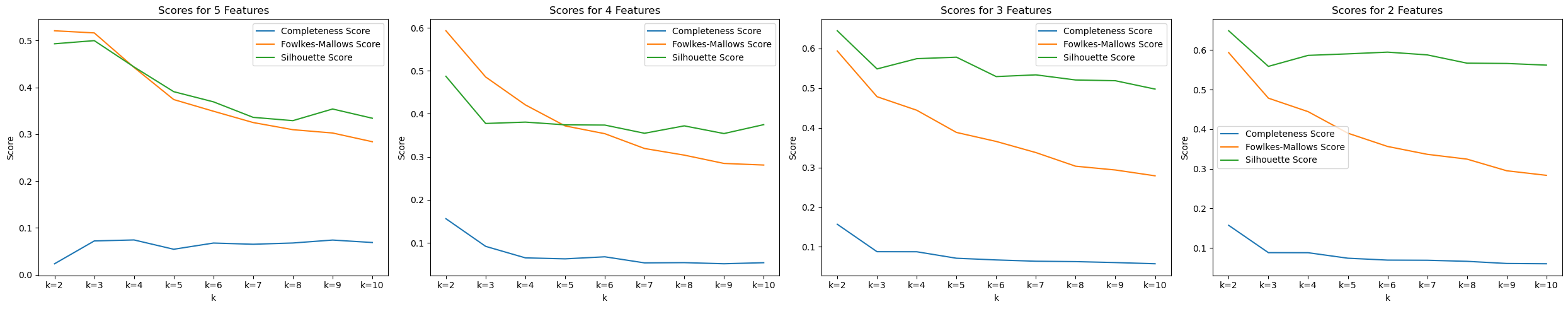
In the image, we were able to see that with 2 features (Fasting Blood Sugar and Resting BP) and 2 clusters, we are able to achieve the best scores : Completeness Score=0.1569, Fowlkes-Mallows Score=0.5934, and Silhouette Score=0.6487.
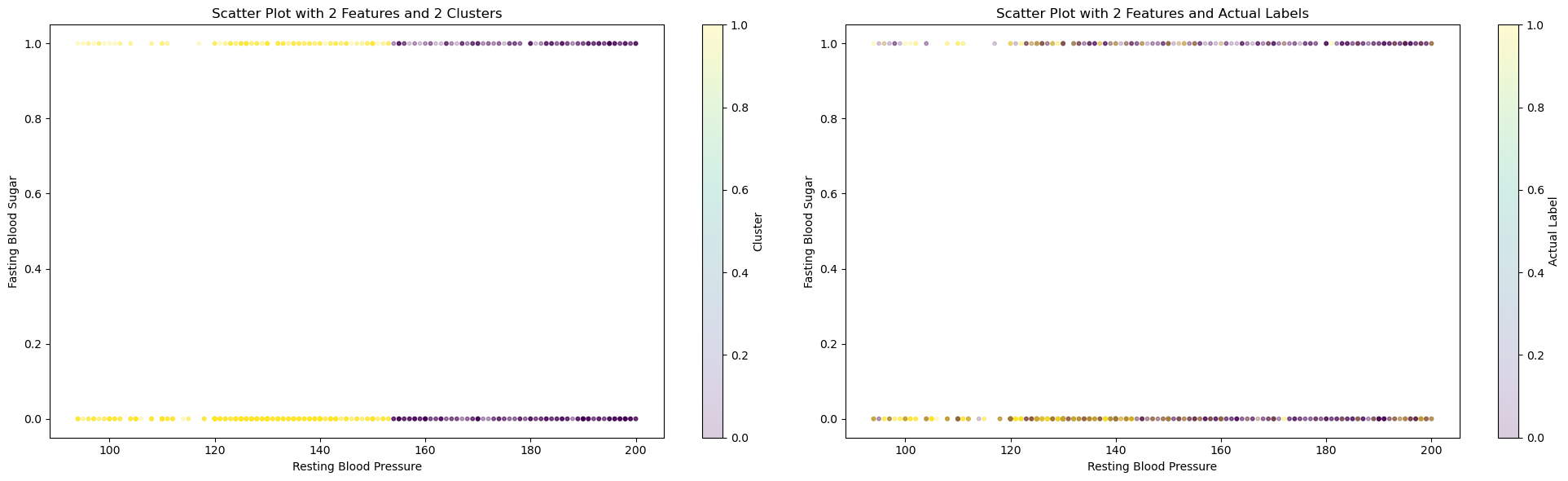
On the left graph, we can see how KMeans cluster the dataset. On the right graph, we can see the actual labels of those data. Data in the middle are hard for KMeans to differentiate, which can explain why all three top scores are still rather low, especially the completeness score, where data points in each cluster are often mixed in the middle range.
Using 2 clusters in KMeans and the given labels, we attempted to see how well the model is able to seperate the labels too. This did not yield very strong results with a 57 percent accuracy obtained using KMeans model. For the future, we can try seperating on a redcuded feature set and try other methods to potentially increase the accuracy.
For next steps, we can potentially experiment with various feature pairs to produce a better KMeans model.
GMM
We used a similar procedure for testing GMM as we did for KMeans. We first tried with different value of number of components in the Gaussian mixture and we found that that it worked best for number of components as 2, which is as expected. We got the following plot for GMM after applying PCA for getting reduced number of features.
For 2 features and 2 components, the scores obtained were: Completeness Score = 0.12 Fowlkes-Mallows Score = 0.583 Silhouette Score = 0.637
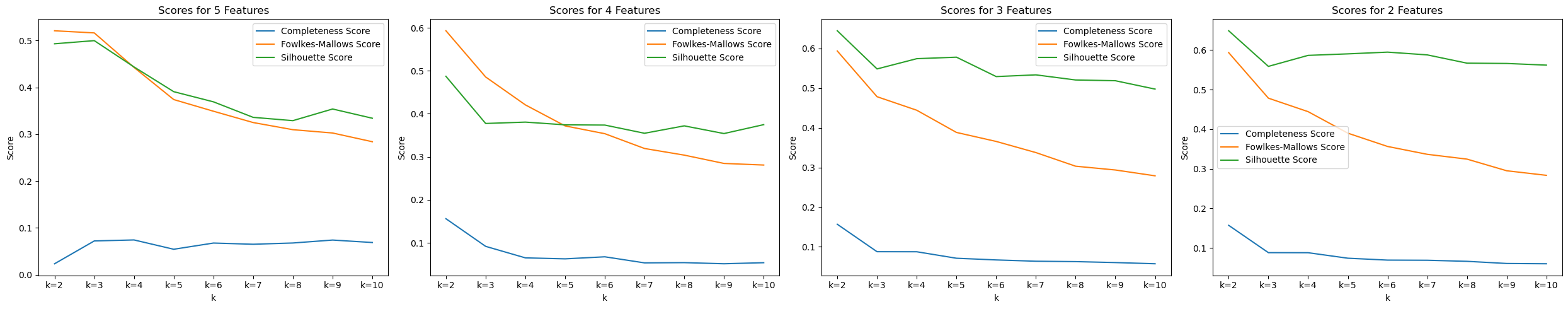
Using 2 components in GMM and the given labels, we attempted to see how well the model is able to seperate the labels too. This did not yield very strong results with a 56 percent accuracy obtained using GMM model. For the future, we can try seperating on a reduced feature set and try other methods to potentially increase the accuracy.
DBSCAN
We used specific methods to estimate the values for epsilon and minimum points. As there are more than 2 features within the dataset, the proper minimum points estimation is double the dimensionality of the dataset.
The optimal epsilon value can be determined from calculating the average distance between each point and its nearest k neighbors, where k = MinPts. From the NearestNeighbors algorithm, plotting the average-k distances in ascending order results in the following graph:

We will select the point of the maximum curvature to estimate the epsilon value. From the graph, the best estimate for epsilon is 21. For the future, we can focus on utilizing the parameters to find the best clustering performance for the DBSCAN algorithm.
Next Steps
For data preprocessing, our next steps are to perform data augmentation so there are more patients represented with no cardiovascular disease. This will make our datset more balanced and prevent overfitting further. We also want to try a supervised method for feature reduction instead of PCA.
For supervised learning algorithms, we plan to run an ensemble learning algorithm such as the Random Forest classifier and see if that improves accuracy/precision/recall. We also want to run logistic regression as it’s well suited for classification.
Lastly, we are lacking accuracy with our unsupervised learning algorithms, with both K-Means and GMM only producing slightly better classification than a random coin toss. Additionally, we are looking into completing the analysis for DBSCAN, since we have only selected the parameter values for the current midterm report. We are hoping to improve these through more research into unsupervised methods for classification tasks with a large number of features.
Timeline
See here for our Gantt Chart.
Contributors
| Name | Contribution |
|---|---|
| Suzan | Data Cleaning, Decision Tree |
| Natasha | Unsupervised Learning |
| Kalp | Supervised, GMM |
| Chih-Chun | K-Means, PCA |
| Eric | PCA, Data cleaning |
References
-
S. Hossain et al., “Machine Learning Approach for predicting cardiovascular disease in Bangladesh: Evidence from a cross-sectional study in 2023 - BMC Cardiovascular Disorders,” BioMed Central, https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-024-03883-2. ↩
-
A. Dinh, S. Miertschin, A. Young, and S. D. Mohanty, “A data-driven approach to predicting diabetes and cardiovascular disease with Machine Learning - BMC Medical Informatics and Decision making,” SpringerLink, https://link.springer.com/article/10.1186/s12911-019-0918-5/metrics. ↩
-
Ogunpola, A.; Saeed, F.; Basurra, S.; Albarrak, A.M.; Qasem, S.N. Machine Learning-Based Predictive Models for Detection of Cardiovascular Diseases. Diagnostics 2024, 14, 144. https://doi.org/10.3390/diagnostics14020144 ↩
-
A. Javaid et al., “Medicine 2032: The Future of Cardiovascular Disease Prevention with Machine Learning and Digital Health Technology,” American Journal of Preventive Cardiology, vol. 12, p. 100379, Dec. 2022. doi:10.1016/j.ajpc.2022.100379. ↩